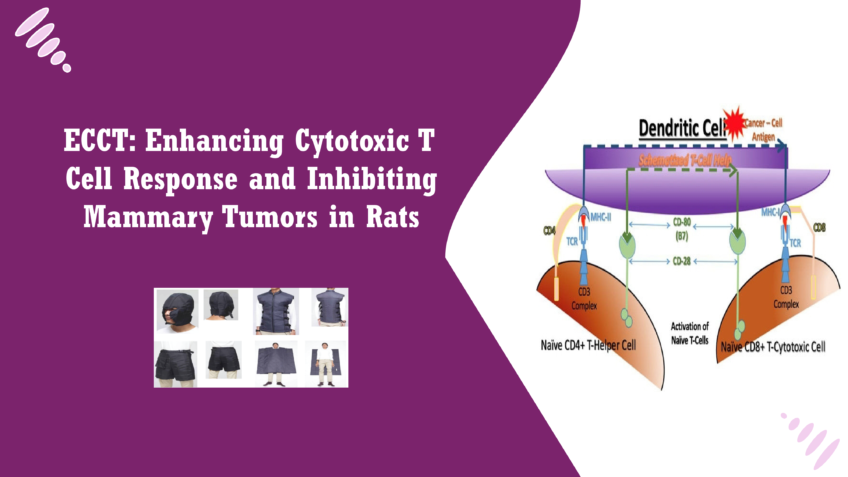
Introduction Breast cancer is one of the most prevalent cancers globally, affecting millions of women and representing a leading cause of cancer-related mortality. In 2020 alone, approximately 2.3 million women were diagnosed with breast cancer, making it the most common cancer worldwide. The disease is particularly concerning due to its complex nature and the myriad factors contributing to its progression, including genetics, lifestyle, and environmental influences. Early detection and advancements in treatment have improved survival rates, yet breast cancer remains a significant health issue, particularly as some patients experience recurrence or metastasis [1]. Despite progress in treatment strategies, existing modalities face numerous challenges. Surgical interventions, such as lumpectomy and mastectomy, while effective in removing tumors, can lead to significant physical and psychological impacts on patients. Systemic therapies, including chemotherapy and targeted therapies, are often accompanied by severe side effects, such as nausea, fatigue, and immunosuppression, which can severely diminish a patient’s quality of life [2]. Furthermore, issues like multi-drug resistance can arise, rendering standard treatments ineffective for some patients. These challenges highlight the urgent need for novel therapeutic approaches that can complement or improve upon current treatment modalities [3]. In response to these needs, researchers are exploring innovative therapies that harness the body’s immune response to combat cancer. One such promising approach is Electro-Capacitive Cancer Therapy (ECCT). This therapy utilizes low-intensity, non-contact electric fields to target cancer cells without directly affecting surrounding healthy tissues. By exposing tumors to carefully controlled electric fields, ECCT aims to disrupt cancer cell proliferation and activate immune responses [4]. Initial studies have shown that ECCT not only inhibits tumor growth but also enhances the activity of cytotoxic T cells, which play a crucial role in the immune system’s ability to eliminate cancerous cells. This novel approach holds the potential to improve outcomes for breast cancer patients while minimizing the adverse effects associated with conventional therapies [5]. As we delve deeper into the research on ECCT, we will explore its mechanisms of action, the results of recent studies, and its implications for the future of cancer treatment. In order to understand the importance of the CD4/CD8 ratio and T cell activation. CD4+ T cells, known as helper T cells, play a vital role in orchestrating the immune response, activating other immune cells, including CD8+ T cells, which are cytotoxic and directly target and kill cancer cells [6]. The ratio of CD4 to CD8 T cells can provide valuable insights into the immune status of an individual, with a higher ratio often indicating a robust immune response. Conversely, a decreased CD4/CD8 ratio can suggest immune suppression, which is commonly seen in cancer patients. Therefore, understanding these dynamics is essential for assessing the effectiveness of ECCT and its impact on the immune landscape in the tumor microenvironment [7]. The experimental designs in the past research on ECCT in breast cancer involved dividing the rats into four groups: a control group that did not receive induction or therapy, a placebo group receiving sham therapy, a group induced with tumors but not treated, and a group that received ECCT. Each group was monitored for tumor growth over a specified period, and this design enabled researchers to draw meaningful comparisons between the effects of ECCT and traditional treatment methods [8]. To measure tumor growth and immune responses, several advanced techniques were employed. Histological examination allowed for the assessment of tumor morphology and cellular changes, while immunohistochemistry was used to identify specific protein expressions related to cell proliferation and apoptosis, such as PCNA (proliferating cell nuclear antigen), ErbB2, and Caspase3 [9]. Additionally, the distributions of immune cells, including CD4+ and CD8+ T cells, were analyzed to gauge the immune response within the tumor environment [10]. The findings from the available research highlighted the significant impact of ECCT on tumor growth rates. Rats treated with ECCT exhibited notably lower tumor growth compared to those in the untreated groups, suggesting that ECCT can effectively inhibit tumor progression. This aligns with the goal of developing non-invasive therapies that can provide similar or enhanced outcomes compared to traditional cancer treatments [11]. In terms of cellular responses, changes in protein expression were observed. A decrease in PCNA and ErbB2 levels in tumors treated with ECCT indicated reduced proliferation and possibly a shift toward apoptotic pathways, as evidenced by increased Caspase3 expression. This highlights ECCT’s potential to not only slow tumor growth but also to induce cancer cell death [12]. Furthermore, observations of T cell distributions revealed an interesting dynamic in the immune response. The treated group showed an increased presence of CD8+ T cells, which are crucial for direct tumor cell elimination [13]. Conversely, the CD4/CD8 ratio was found to be lower in the ECCT-treated group compared to untreated counterparts, suggesting a shift in immune activity that may favor more aggressive targeting of the tumor [14]. These findings underscore the importance of integrating immune response assessments into cancer therapy research, particularly for innovative approaches like ECCT. As the scientific community continues to explore the intricacies of cancer treatment, understanding the relationship between therapies, immune activation, and tumor dynamics will be crucial in developing more effective strategies for managing breast cancer and other malignancies [15]. The results from multiple studies reveal significant insights into the effects of Electro-Capacitive Cancer Therapy (ECCT) on mammary tumors and the underlying immune mechanisms. The observed reduction in tumor proliferation, coupled with an increase in apoptosis, highlights the potential of ECCT as an effective therapeutic strategy against breast cancer [16]. Interpretation of Reduced Tumor Proliferation and Increased Apoptosis The decrease in tumor proliferation, as indicated by lower levels of PCNA and ErbB2, suggests that ECCT effectively disrupts the mechanisms driving cancer cell growth. This is particularly noteworthy because ErbB2 is often overexpressed in aggressive breast cancers and is associated with poor prognosis. By targeting the proliferation pathways, ECCT may help slow down tumor progression and potentially prevent metastasis [17]. Increased apoptosis, marked by elevated Caspase3 levels, further reinforces the therapeutic potential of ECCT. Apoptosis is a critical process in eliminating dysfunctional cells,