Electro-Capacitive Cancer Therapy (ECCT) is a cutting-edge, non-invasive treatment that harnesses the power of alternating electric fields to target and disrupt cancer cell proliferation. Unlike conventional treatments like chemotherapy or radiation, ECCT offers a novel approach by applying external electric fields to tumors, which may selectively interfere with cancer cell division while leaving healthy cells relatively unaffected.
The growing interest in ECCT stems from its potential to inhibit tumor growth without causing the widespread damage typically seen with traditional cancer treatments. Studies have shown that ECCT can reduce tumor proliferation by inducing apoptosis (programmed cell death) and inhibiting key signaling pathways involved in cancer growth. In addition, ECCT’s non-invasive nature makes it an attractive option for patients seeking alternatives to more aggressive therapies [1].
How ECCT works is based on the application of alternating current electric fields (AC-EF) between two capacitive electrodes positioned near the tumor. These electric fields are applied at specific frequencies and intensities designed to exploit the unique electrical properties of cancer cells. Cancer cells have different dielectric properties compared to normal cells, particularly during mitosis (cell division). By applying alternating electric fields at intermediate frequencies, ECCT can destabilize the cancer cells’ membrane integrity, interrupting mitosis and leading to cell death. This effect is particularly important in slowing tumor growth and preventing metastasis.
Role of CCL2 and IL18 in Cancer
In the context of cancer progression, Cytokines such as CCL2 and IL18 play critical roles in promoting inflammation and influencing tumor behavior. These proteins are essential for cell signaling, and their overexpression can lead to the proliferation, migration, and survival of cancer cells. Understanding how these cytokines interact with ECCT is key to grasping the therapeutic potential of this treatment [2].CCL2: A Key Player in Cancer Metastasis
CCL2 (C-C motif chemokine ligand 2) is a chemokine that is heavily involved in the recruitment of monocytes to sites of inflammation and injury. In the context of cancer, it is known to promote metastasis, particularly in breast cancer. CCL2 aids in the migration of immune cells, such as macrophages, to the tumor microenvironment, where they can support tumor growth and metastasis by enhancing the inflammatory response. It also promotes angiogenesis (the formation of new blood vessels), which tumors rely on for nutrients and oxygen. In breast cancer, CCL2 has been associated with a higher propensity for metastatic spread, especially to distant organs like the lungs. This makes it a crucial target for therapies aiming to control tumor growth and metastasis. By reducing CCL2 expression, treatments like ECCT may help suppress the tumor’s ability to grow and invade other tissues [3].IL18: Modulating the Tumor Immune Microenvironment
Interleukin-18 (IL18) is another cytokine that plays a significant role in cancer progression, particularly in modulating the immune response. It is known to influence the activity of macrophages, a type of immune cell that can either promote or inhibit tumor growth depending on the signals they receive. IL18 has been shown to facilitate a pro-tumor immune environment by promoting macrophage polarization towards a tumor-supportive phenotype, which contributes to excessive angiogenesis—the formation of new blood vessels that feed tumor cells. IL18 can drive inflammation in the tumor microenvironment, leading to enhanced tumor survival and growth. By increasing macrophage activity and angiogenesis, IL18 essentially creates conditions that allow tumors to thrive. In the context of breast cancer, IL18 has been linked to more aggressive tumor behavior and poorer prognosis [4]. In studies involving ECCT, it has been observed that exposure to electric fields may downregulate the expression of IL18, thus reducing its ability to promote tumor growth and angiogenesis. This suggests that one of ECCT’s anti-tumor mechanisms may involve suppressing the inflammatory and immune-modulating effects of cytokines like IL18, which are otherwise supportive of cancer progression. Together, CCL2 and IL18 represent critical targets in cancer therapy, particularly in treatments aimed at controlling tumor growth and metastasis. By regulating the expression of these cytokines, therapies such as ECCT may not only halt cancer proliferation but also modulate the immune environment to be less favorable for cancer development. This dual action direct inhibition of cancer cells and modification of the tumor microenvironment makes ECCT a promising approach in the fight against cancer [5].
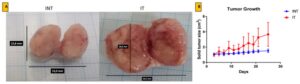
Growth of solid breast cancer mass after ECCT exposure treatment.
The Experiment: How ECCT Affects Breast Tumor Growth
In this study, researchers investigated how Noncontact Electro-Capacitive Cancer Therapy (ECCT) impacts breast tumor growth in a controlled experiment using rats. The animal model involved inducing breast tumors in rats with a carcinogen known as 7,12-dimethylbenz[a]anthracene (DMBA), which reliably causes breast cancer in rodents, making it a suitable model for studying human breast cancer. The experiment was designed with four distinct groups of rats:- NINT (Non-induced, no treatment): Rats that were not exposed to DMBA and did not receive ECCT treatment. This served as the healthy control group.
- NIT (Non-induced, treatment): Rats that were not exposed to DMBA but were treated with ECCT. This group helped to observe any effects of ECCT on non-cancerous tissue.
- INT (Induced, no treatment): Rats that were induced with breast tumors via DMBA but did not receive ECCT. This group allowed for the observation of the natural progression of tumor growth.
- IT (Induced, treatment): Rats that were induced with breast tumors via DMBA and treated with ECCT. This group was the focus of the study, allowing the researchers to observe how ECCT influences tumor growth.
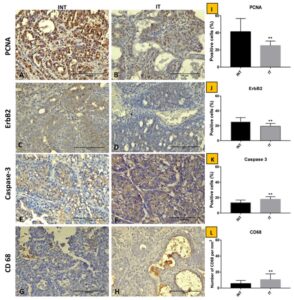
- PCNA (Proliferating Cell Nuclear Antigen): A marker of cell proliferation, which indicates the rate at which cancer cells are dividing and growing.
- ErbB2 (also known as HER2): A protein involved in the growth and progression of certain aggressive types of breast cancer.
- Caspase3: A protein that plays a key role in the process of apoptosis (programmed cell death), indicating the death of cancer cells.
- CD68: A marker for macrophages, which are immune cells that can either promote or inhibit tumor growth, depending on their activity in the tumor microenvironment.
- PCNA is a direct indicator of how quickly cancer cells are dividing. Lower levels of PCNA in the ECCT-treated rats indicate that tumor cell proliferation had slowed down, meaning fewer cancer cells were being produced.
- ErbB2 is associated with aggressive tumor growth, particularly in breast cancer. The reduction of ErbB2 expression suggests that ECCT may also reduce the aggressiveness of the tumors, making them less likely to grow rapidly or metastasize.
- Caspase3 expression was elevated in the ECCT-treated group.
- CD68 levels were also increased in the ECCT-treated tumors.

Immunostaining of breast adenocarcinoma tissue sections after ECCT treatment.
Downregulation of CCL2 and IL18
CCL2 and IL18, two cytokines known to promote cancer progression, were significantly downregulated in the ECCT-treated tumors. CCL2 is associated with metastasis, particularly in breast cancer, as it recruits immune cells that can promote tumor growth. By reducing CCL2 expression, ECCT may help to prevent the spread of cancer to other parts of the body. IL18 plays a role in enhancing angiogenesis (the formation of new blood vessels) within tumors, which allows them to grow and thrive. The downregulation of IL18 in ECCT-treated tumors suggests that ECCT may help to starve tumors by reducing their blood supply [9]. These findings indicate that ECCT not only slows down tumor growth but also alters the tumor microenvironment in a way that makes it less conducive to cancer progression. By reducing the expression of key proteins involved in cancer proliferation and survival, ECCT could offer a promising new avenue for cancer treatment that works through both direct anti-tumor effects and immune modulation. These findings lend substantial support to the idea that Noncontact Electro-Capacitive Cancer Therapy (ECCT) can exert a potent anti-proliferative effect on tumors. The study demonstrated that ECCT significantly reduced tumor proliferation by decreasing key markers like PCNA and ErbB2 in treated rats. This means that fewer cancer cells were able to divide and grow, slowing the progression of the tumors. Additionally, the observed increase in Caspase3 suggests that ECCT promotes apoptosis (programmed cell death), further reducing tumor size by eliminating cancer cells. The enhanced presence of CD68, a marker for immune cells like macrophages, indicates that ECCT may also enhance immune response, potentially adding another layer of defence against tumor growth [10]. One of the critical observations from the study is the downregulation of cytokines such as CCL2 and IL18 in ECCT-treated tumors. Both of these cytokines are closely linked to cancer progression: CCL2 is known to promote metastasis, particularly in breast cancer, by recruiting immune cells that support tumor growth. Its downregulation suggests that ECCT may help inhibit metastasis and reduce the risk of cancer spreading to other parts of the body. IL18 contributes to angiogenesis (the formation of blood vessels that supply nutrients to tumors), allowing cancer to grow. By downregulating IL18, ECCT might limit tumor blood supply, effectively “starving” the tumor [11]. When compared to the untreated groups, where tumor proliferation markers like PCNA and ErbB2 remained elevated, these findings strongly support ECCT’s therapeutic potential. In the untreated rats with DMBA-induced tumors, the upregulation of CCL2 and IL18 further underscores the role of these cytokines in facilitating tumor growth and spread. ECCT’s ability to downregulate these signals positions it as a novel cancer treatment that may specifically target the tumor microenvironment. The results of this study pave the way for ECCT to be considered a non-invasive alternative for controlling tumor growth. By inhibiting cell proliferation and enhancing the immune response, ECCT could offer an effective treatment that doesn’t rely on surgery, chemotherapy, or radiation. This could be particularly beneficial for patients who are unable to tolerate more aggressive treatments or are looking for therapies with fewer side effects [12]. However, to fully understand ECCT’s potential, more research is necessary. There is a need for further exploration of the molecular mechanisms by which ECCT exerts its effects on different types of cancer. This study focused on breast cancer in rats, but additional studies should examine how ECCT impacts other cancers, such as lung, colon, or brain tumors, to determine its broader applicability. Moreover, a deeper understanding of the role that cytokines like CCL2 and IL18 play in ECCT’s anti-tumor effects could lead to more targeted therapies. Cytokines are critical regulators of the tumor microenvironment, and manipulating their levels with ECCT could provide new strategies for cancer management. By studying how ECCT affects cytokine signaling pathways, researchers could refine and optimize the therapy, making it even more effective across a range of cancer types [13].Conclusion
In summary, this research highlights the promising potential of ECCT in regulating tumor proliferation by modulating cytokine expression and promoting cell death in cancer cells. The downregulation of CCL2 and IL18, coupled with the reduction in tumor proliferation markers, demonstrates that ECCT may serve as a powerful tool for cancer treatment. Its ability to alter the tumor microenvironment without the need for invasive procedures positions ECCT as a valuable addition to the arsenal of cancer therapies. As the field of non-invasive cancer treatments grows, ECCT’s role will continue to evolve. Further exploration of its applications and mechanisms is essential to maximize its therapeutic benefits. Understanding how cytokine modulation contributes to ECCT’s efficacy could open new avenues for personalized cancer treatments, potentially revolutionizing how we approach cancer care.References
- Alamsyah F, Ajrina IN, Dewi FNA, et al.: Antiproliferative Effect of Electric Fields on Breast Tumor Cells In Vitro and In Vivo. Indones J Cancer Chemoprevent. 2015; 6(3): 7
- Mujib SA, Alamsyah F, Taruno WP: Cell Death and Induced p53 Expression in Oral Cancer, HeLa, and Bone Marrow Mesenchyme Cells under the Exposure to Noncontact Electric Fields. Integr Med Int. 2017; 4(3–4): 161–70.
- Kirson ED, Gurvich Z, Schneiderman R, et al.: Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004; 64(9): 3288–95.
- Kirson ED, Dbalý V, Tovarys F, et al.: Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007; 104(24): 10152–7
- Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells. Sci Rep. 2015; 5: 18046.
- Timaner M, Beyar-Katz O, Shaked Y: Analysis of the Stromal Cellular Components of the Solid Tumor Microenvironment Using Flow Cytometry. Curr Protoc Cell Biol. 2016; 70: 19.8.1–19.18.12
- Kitamura T, Qian BZ, Soong D, et al.: CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasisassociated macrophages. J Exp Med. 2015; 212(7): 1043–59.
- Kobori T, Hamasaki S, Kitaura A, et al.: Interleukin-18 Amplifies Macrophage Polarization and Morphological Alteration, Leading to Excessive Angiogenesis. Front Immunol. 2018; 9: 334.
- Park JI, Song KH, Jung SY, et al.: Tumor-Treating Fields Induce RAW264.7 Macrophage Activation Via NK-κB/MAPK Signaling Pathways. Technol Cancer Res Treat. 2019; 18: 1533033819868225.
- Yang Y, Cheon S, Jung MK, et al.: Interleukin-18 enhances breast cancer cell migration via down-regulation of claudin-12 and induction of the p38 MAPK pathway. Biochem Biophys Res Commun. 2015; 459(3): 379–86.
- Menegatti S, Bianchi E, Rogge L: Anti-TNF Therapy in Spondyloarthritis and Related Diseases, Impact on the Immune System and Prediction of Treatment Responses. Front Immunol. 2019; 10: 382.
- Yu PF, Huang Y, Han YY, et al.: TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene. 2017; 36(4): 482–490.
- Taki FA, Abdel-Rahman AA, Zhang B: A comprehensive approach to identify reliable reference gene candidates to investigate the link between alcoholism and endocrinology in Sprague-Dawley rats. PLoS One. 2014; 9(5): e94311.

