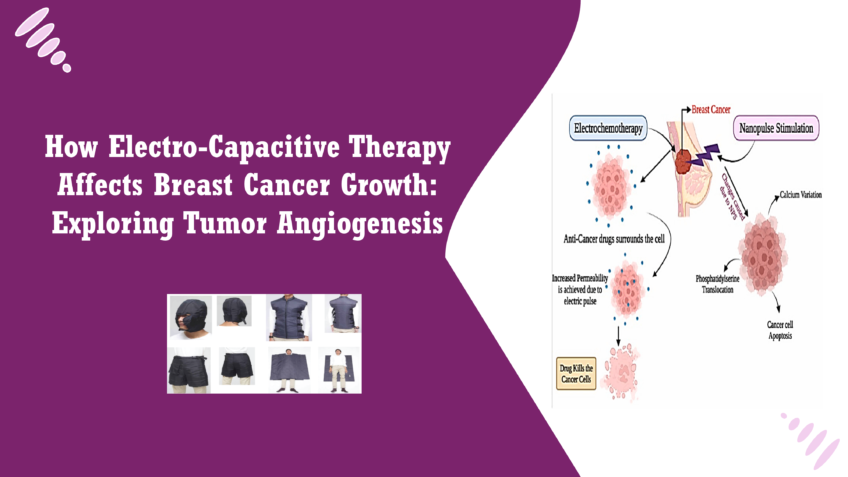
Electro-Capacitive Therapy (ECT) is an innovative medical treatment that employs non-contact alternating electric fields to target and disrupt cancer cell activity. Unlike traditional cancer treatments, which often involve invasive procedures like surgery or systemic therapies such as chemotherapy and radiation, ECT provides a non-invasive approach to cancer care. By using electric fields that can penetrate tissues without direct contact, ECT minimizes physical trauma to the body and reduces the potential for adverse side effects associated with conventional treatments.
ECT operates on the principle of capacitive coupling, where electric fields are generated by electrodes placed outside the body. These fields interact with cancer cells, selectively disrupting their normal function, particularly their ability to divide and grow. The non-invasive nature of ECT makes it an appealing option for patients who may not tolerate more aggressive treatments well, allowing for a gentler alternative that aims to reduce cancer progression while preserving the integrity of healthy tissues [1].
How ECT is Used in Cancer Treatment
ECT is emerging as a potential adjunct therapy in the treatment of various types of cancer. Its application is based on the understanding that cancer cells often exhibit altered electrical properties compared to normal cells. By harnessing these differences, ECT aims to selectively target and impair cancer cell proliferation without significantly affecting surrounding healthy tissues. In clinical settings, ECT can be administered alongside other treatments, such as chemotherapy or radiation, to enhance their effectiveness while potentially reducing their side effects. The therapy is administered over a specified duration, during which patients are exposed to controlled electric fields that interact with their tumors. This method not only addresses the cancer cells directly but may also influence the tumor microenvironment, thereby impacting factors such as blood vessel formation, known as angiogenesis, which plays a crucial role in tumor growth and metastasis [2].Why Angiogenesis (Formation of New Blood Vessels) is Important in Cancer Growth
Angiogenesis is the physiological process through which new blood vessels form from pre-existing ones. In the context of cancer, angiogenesis is vital for tumor growth and survival. As tumors expand, they require an adequate supply of oxygen and nutrients to sustain their metabolic needs. The process of angiogenesis allows tumors to develop their own blood supply, enabling them to grow larger and potentially spread to other parts of the body (metastasis). Tumors often release specific signaling molecules, such as Vascular Endothelial Growth Factor (VEGF), that stimulate nearby blood vessels to grow towards the tumor. This creates a network of blood vessels that not only nourishes the tumor but also facilitates the dissemination of cancer cells into the bloodstream, leading to metastasis. Targeting angiogenesis has become an important therapeutic strategy in cancer treatment. By inhibiting the formation of new blood vessels, it is possible to starve tumors of the necessary resources they need to grow and spread. Consequently, understanding how ECT affects angiogenesis could provide valuable insights into its role as a therapeutic option in cancer treatment, particularly in reducing tumor growth and improving patient outcomes [3]. Blood vessels play a critical role in supporting the growth and survival of tumors. As tumors develop, they undergo significant metabolic changes that increase their demand for oxygen and nutrients. Without a sufficient blood supply, tumors cannot grow beyond a certain size because they would not receive the necessary elements to support their rapid proliferation. Therefore, the formation of new blood vessels, a process known as angiogenesis, is essential for tumor growth and progression. Blood vessels not only supply the tumor with oxygen and nutrients but also facilitate the removal of waste products generated during cellular metabolism. In cancer, the structure and function of blood vessels can be significantly altered. Tumor-associated blood vessels are often irregular, leaky, and poorly organized, leading to an abnormal distribution of blood flow within the tumor. This aberrant vasculature can create a hypoxic environment, where certain regions of the tumor become oxygen-deficient, driving further angiogenic signaling to recruit more blood vessels [4]. Tumors utilize the process of angiogenesis to secure a constant supply of nutrients and oxygen essential for their growth and survival. As the tumor expands, it secretes various pro-angiogenic factors, the most notable being Vascular Endothelial Growth Factor (VEGF). These factors stimulate surrounding endothelial cells to proliferate and migrate, forming new blood vessels that infiltrate the tumor. Once the new blood vessels are established, they facilitate the transport of oxygen, glucose, and other nutrients into the tumor microenvironment, thereby supporting its growth. Additionally, the newly formed vasculature provides a pathway for cancer cells to enter the bloodstream, allowing them to disseminate and form secondary tumors (metastases) in distant organs. This capacity to adapt and manipulate the blood supply is a hallmark of cancer, making angiogenesis a critical factor in tumor progression [5].The Significance of Targeting Angiogenesis in Cancer Therapies
Given the pivotal role of angiogenesis in tumor growth and metastasis, targeting this process has emerged as a promising therapeutic strategy in cancer treatment. Anti-angiogenic therapies aim to inhibit the formation of new blood vessels, effectively starving the tumor of the nutrients and oxygen it requires to grow. Several approaches have been developed to disrupt angiogenesis. These include the use of monoclonal antibodies that target VEGF (such as Bevacizumab) and small-molecule tyrosine kinase inhibitors that block the signaling pathways involved in angiogenesis. By preventing tumors from establishing a robust blood supply, these therapies can slow tumor growth, reduce the likelihood of metastasis, and potentially enhance the effectiveness of other treatments, such as chemotherapy or radiation. Furthermore, understanding the mechanisms of angiogenesis can help identify biomarkers that predict tumor response to anti-angiogenic therapies, allowing for more personalized and effective treatment strategies. The ability to target angiogenesis not only provides a novel avenue for cancer therapy but also underscores the interconnectedness of the tumor and its microenvironment, highlighting the need for comprehensive treatment approaches that consider both the cancer cells and the supporting structures that enable their growth [6].How ECT Impacts Tumour Angiogenesis
Electro-Capacitive Therapy (ECT) operates by generating alternating electric fields (AEFs) that interact with cancer cells. This non-invasive technique utilizes capacitive coupling, where electrodes placed externally on the body produce electric fields that penetrate the tissue without direct contact. The alternating electric fields influence the electrical properties of the cancer cells, leading to changes in their physiological behavior. The application of ECT results in the alteration of cellular processes, including cell proliferation, apoptosis (programmed cell death), and cellular communication. Specifically, these electric fields can disrupt the mechanisms by which cancer cells divide and grow, thereby impairing their ability to form and maintain a supportive microenvironment, which includes angiogenesis. By targeting the aberrant signaling pathways in cancer cells, ECT may effectively inhibit tumor growth while promoting an environment that could lead to changes in blood vessel formation associated with tumor development [7].How ECT Affects Blood Vessel Formation in Tumors
Recent studies have investigated the impact of ECT on angiogenesis in tumor environments. One key area of research focuses on how the application of ECT can influence the signaling pathways that regulate blood vessel formation. In particular, researchers are interested in understanding whether ECT can inhibit or promote angiogenesis depending on the context of treatment and the tumor type. In preclinical models, such as studies conducted on rat models with breast tumors induced by chemical agents like 7,12-dimethylbenz(α)anthracene (DMBA), ECT was applied for a specific duration and frequency. The goal was to observe any changes in the tumor microenvironment, especially regarding the formation and density of blood vessels within the tumor tissue. By analyzing various markers of angiogenesis, including the expression of genes and proteins related to blood vessel development, researchers aimed to elucidate the mechanisms through which ECT could alter the tumor’s angiogenic capacity.Increased Blood Vessel Formation Linked to Specific Gene Activity (Vegfr2)
One of the pivotal findings from the research on ECT’s impact on angiogenesis is the observed increase in blood vessel formation linked to the activity of specific genes, particularly Vascular Endothelial Growth Factor Receptor-2 (Vegfr2). In the studies, exposure to ECT led to a significant upregulation of Vegfr2 expression in the tumor tissues, suggesting that ECT may stimulate angiogenesis through this receptor’s signaling pathway. While the increased expression of Vegfr2 is often associated with enhanced blood vessel formation, it is important to note that the dynamics can be complex. For instance, the study found no corresponding increase in the primary angiogenic signal, Vascular Endothelial Growth Factor-A (Vegfa), which typically drives angiogenesis. This indicates that ECT may alter the angiogenic landscape in a way that promotes blood vessel formation while potentially regulating or balancing other factors involved in the process [8]. These findings highlight the dual role ECT may play in tumor angiogenesis, where it could potentially enhance blood vessel formation through specific signaling pathways while disrupting the overall tumor growth and survival mechanisms. Understanding these interactions is critical for developing ECT as a therapeutic strategy, as it sheds light on how electrical fields can be harnessed to modify tumor behavior and improve treatment outcomes in cancer patients.: In a recent study investigating the effects of Electro-Capacitive Therapy (ECT) on breast cancer, researchers employed a rat model where breast tumors were induced using the chemical carcinogen 7,12-dimethylbenz(α)anthracene (DMBA). The objective of the study was to evaluate how non-contact ECT affects both normal breast tissue and tumor angiogenesis. For this purpose, the rats were exposed to ECT, utilizing alternating electric fields generated at a frequency of 150 kHz and a peak-to-peak voltage of 18 V for a duration of 10 hours per day over a 21-day treatment period. The study aimed to analyze various biological parameters, including the density of blood vessels in tumor tissues and the expression of key genes associated with angiogenesis. The methodology included the use of immunohistochemistry to assess blood vessel formation and quantitative PCR (qPCR) to measure gene expression related to angiogenic signaling. The results of the study revealed significant insights into the effects of ECT on tumor and normal breast tissues. Notably, ECT exposure did not lead to any observable changes in the angiogenesis of normal breast tissue, indicating that ECT may selectively target cancerous tissues without adversely affecting healthy cells. This selectivity is a crucial advantage of ECT, as it minimizes damage to surrounding healthy tissue, potentially reducing side effects typically associated with more aggressive treatments [9]. In contrast, tumors exposed to ECT exhibited a marked increase in blood vessel formation. The study found that ECT significantly promoted angiogenesis in tumor tissues, suggesting that the electrical fields may stimulate processes related to new blood vessel development specifically within the tumor microenvironment. This observation highlights ECT’s potential role in modifying the tumor’s vascular architecture, which is essential for sustaining its growth.How ECT Influenced Gene Expression Related to Blood Vessel Formation
One of the key mechanisms through which ECT impacts angiogenesis is its influence on gene expression related to blood vessel formation. In the study, the expression of Vascular Endothelial Growth Factor Receptor-2 (Vegfr2), a crucial player in angiogenic signaling, was significantly upregulated in the tumor tissues subjected to ECT. This suggests that ECT may enhance the signaling pathways responsible for angiogenesis by promoting the expression of Vegfr2. Interestingly, the study indicated no significant changes in the expression of Vascular Endothelial Growth Factor-A (Vegfa), which typically acts as the primary angiogenic factor. This finding raises important questions about the specific pathways activated by ECT and how they differ from conventional angiogenic processes. The increased expression of Vegfr2, despite the unchanged levels of Vegfa, implies a potential alternative pathway through which ECT may drive angiogenesis, highlighting the complexity of tumor biology. The findings from this study underscore the potential of ECT as a targeted therapeutic strategy in breast cancer treatment. By selectively enhancing angiogenesis within tumors while sparing normal tissues, ECT may provide a novel approach to disrupt tumor growth. The ability to promote blood vessel formation specifically in the tumor microenvironment could facilitate the delivery of additional therapeutic agents, enhancing their efficacy while minimizing systemic toxicity. Moreover, by modulating the tumor’s vascular network, ECT could play a role in improving the overall therapeutic landscape for patients, particularly when used in conjunction with other treatment modalities. For example, combining ECT with chemotherapy may enhance drug delivery to tumor sites by ensuring that blood vessels are appropriately developed, thus improving the distribution of therapeutic agents. While the initial findings of this study are promising, further research is essential to comprehensively understand the mechanisms underlying ECT’s effects on tumor angiogenesis and overall cancer treatment. Future studies should explore the optimal parameters for ECT application, including duration, frequency, and intensity, to maximize therapeutic benefits while minimizing any potential adverse effects [10]. Additionally, research should aim to clarify the signaling pathways activated by ECT and their implications for other key processes in cancer progression, such as metastasis and resistance to therapies. Investigating the long-term effects of ECT on tumor growth, patient outcomes, and quality of life will also be vital in establishing ECT as a standard component of cancer treatment protocols. By promoting vascular changes in tumors through mechanisms such as the upregulation of Vascular Endothelial Growth Factor Receptor-2 (Vegfr2), ECT not only aids in understanding the complex interactions between cancer cells and their microenvironment but also opens new avenues for therapeutic interventions. The future of cancer treatment is increasingly leaning towards non-invasive therapies, and ECT exemplifies this shift. As a non-contact treatment modality, ECT minimizes the risks associated with traditional interventions, such as surgery or radiation therapy, thereby enhancing patient comfort and safety. By integrating ECT into existing treatment regimens, there is potential to improve overall therapeutic outcomes while reducing side effects commonly associated with conventional cancer treatments. Moreover, the ability of ECT to selectively influence tumor behavior raises the possibility of personalized cancer therapy approaches. By tailoring ECT parameters based on individual tumor characteristics, clinicians may optimize treatment efficacy and further enhance patient quality of life. The promise of non-invasive therapies like ECT signals a transformative change in how we approach cancer management, moving towards more holistic and patient-centered care. Despite the encouraging findings surrounding ECT, much remains to be understood about its long-term effects and broader applications in cancer therapy. Future research should focus on conducting comprehensive clinical trials to validate ECT’s efficacy across different cancer types and stages, as well as to determine optimal treatment protocols. Investigating the potential synergistic effects of ECT when combined with other cancer therapies, such as chemotherapy and immunotherapy, could unlock new therapeutic strategies that enhance patient outcomes. Additionally, understanding the biological mechanisms driving ECT’s effects will be crucial for refining this approach and ensuring its successful integration into standard cancer care practices. In conclusion, Electro-Capacitive Therapy represents a promising frontier in cancer treatment, with its ability to target both tumor growth and angiogenesis. As research continues to unfold, ECT has the potential to significantly impact the future of cancer therapy, offering hope for more effective, less invasive treatment options that prioritize patient well-being. Continued investment in research and clinical exploration of ECT will be essential to unlocking its full therapeutic potential and improving the lives of those affected by cancer.References
- Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR. 2016. VEGFA/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 10(4):347–354. doi:10.1007/s1207901603528.
- Alamsyah F, Ajrina IN, Nur F, Dewi A, Iskandriati D, Prabandari SA, Taruno WP. 2015. Antiproliferative effect of electric fields on breast tumor cells in vitro and in vivo. Indones. J. Cancer Chemoprevention 6(3):71– 77.
- Alamsyah F, Fadhlurrahman A, Pello J, Firdausi N, Evi S, Karima F, Pratiwi R, Fitria L, Nurhidayat L, Taruno W. 2018. PO111 Noncontact electric fields inhibit the growth of breast cancer cells in animal models and induce local immune reaction. ESMO Open 3:A269. doi:10.1136/esmoopen2018eacr25.636.
- Alamsyah F, Pratiwi R, Firdausi N, Irene Mesak Pello J, Evi Dwi Nugraheni S, Ghitha Fadhlurrahman A, Nurhidayat L, Purwo Taruno W. 2021. Cytotoxic T cells response with decreased CD4/CD8 ratio during mammary tumors inhibition in rats induced by noncontact electric fields. F1000Research 10:35. doi:10.12688/f1000research.27952.1.
- Chen Y, Ye L, Guan L, Fan P, Liu R, Liu H, Chen J, Zhu Y, Wei X, Liu Y, Bai H. 2018. Physiological electric field works via the VEGF receptor to stimulate neovessel formation of vascular endothelial cells in a 3D environment. Biol. Open 7(9):bio035204. doi:10.1242/bio.035204.
- Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell 100(1):57–70. doi:10.1016/S0092 8674(00)816839. Karar J, Maity A. 2011. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 4:1–8. doi:10.3389/fnmol.2011.00051.
- Kim EH, Song HS, Yoo SH, Yoon M. 2016. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget 7(40):65125. doi:10.18632/oncotarget.11372.
- Vellingiri B, Iyer M, Subramaniam MD, Jayaramayya K, Siama Z, Giridharan B, Narayanasamy A, Dayem AA, Cho SG. 2020. Understanding the role of the transcription factor Sp1 in ovarian cancer: From theory to practice. Int. J. Mol. Sci. 21(3):1153. doi:10.3390/ijms21031153.
- Wagner M, Wiig H. 2015. Tumor interstitial fluid formation, characterization, and clinical implications. Front. Oncol. 5(MAY):1–12. doi:10.3389/fonc.2015.00115.
- Zimna A, Kurpisz M. 2015. HypoxiaInducible factor 1 in physiological and pathophysiological angiogenesis: Applications and therapies. Biomed Res. Int. 2015:549412. doi:10.1155/2015/549412.