Introduction
Cancer remains one of the most pressing health challenges globally, affecting millions of individuals each year. Traditional treatment modalities, including surgery, chemotherapy, and radiation therapy, have been cornerstones in the fight against cancer. However, these approaches often come with significant limitations, such as severe side effects, potential for recurrence, and the development of resistance in cancer cells. Patients frequently experience adverse reactions that can diminish their quality of life, highlighting the urgent need for innovative and less invasive therapeutic options [1]. In response to these challenges, researchers and clinicians are exploring novel therapeutic strategies that harness the body’s biological responses to fight cancer more effectively. One such promising approach is Noncontact Electro Capacitive Cancer Therapy (ECCT). This innovative treatment utilizes low-intensity alternating electric fields to disrupt cancer cell proliferation while minimizing damage to surrounding healthy tissue [2]. Unlike traditional therapies that directly target the tumor, ECCT works from a distance, offering a non-invasive alternative that may reduce the risk of side effects. ECCT has garnered attention for its potential to activate specific cellular pathways that induce apoptosis—programmed cell death—without harming normal cells. Initial studies have shown that this method can significantly hinder tumor growth, making it a subject of interest in preclinical and clinical research [3]. As scientists continue to investigate the molecular mechanisms underlying ECCT, its role in modulating inflammatory markers such as CCL2 and IL18 offers valuable insights into how this therapy could enhance anti-tumor efficacy and reshape the future of cancer treatment. This blog will explore the findings of recent research on ECCT, shedding light on its potential benefits and implications for patients battling cancer [4].Understanding ECCT
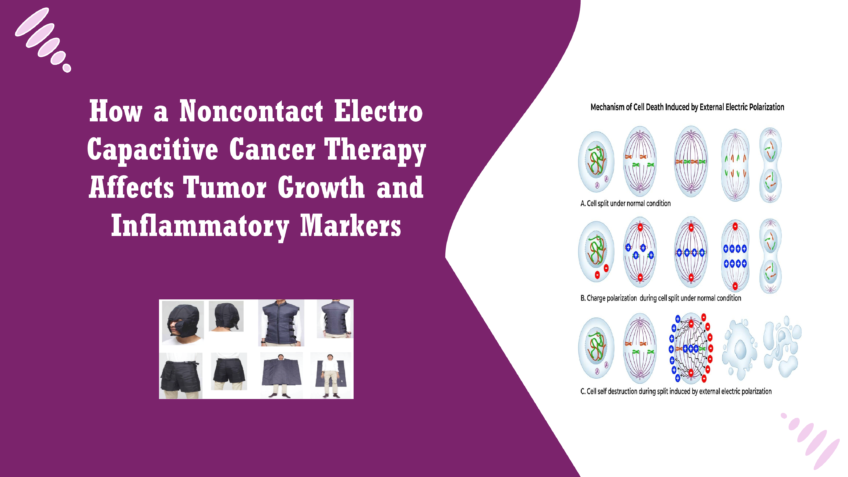
Noncontact Electro Capacitive Cancer Therapy (ECCT) operates on the principle of utilizing low-intensity alternating electric fields to disrupt the biological processes of cancer cells. The therapy involves placing capacitive electrodes around the treatment area, creating an electric field that penetrates tissues without direct contact. This non-invasive approach allows for the modulation of cellular activities through the application of specific electric frequencies [5] When cancer cells are exposed to these alternating electric fields, several biological responses can be triggered. The primary mechanism appears to be the induction of apoptosis, a form of programmed cell death that is crucial for eliminating damaged or dysfunctional cells. ECCT has been shown to disrupt the normal mitotic process of cancer cells, causing them to halt their division and ultimately die. This disruption is thought to occur through several pathways, including the destabilization of microtubules during mitosis and the activation of apoptotic signalling cascades[6]. Additionally, ECCT may influence the tumour microenvironment by modulating the activity of surrounding immune cells. Electric fields can impact macrophage function, enhancing their ability to recognize and eliminate cancer cells [7]. This interaction is crucial because a supportive immune response can significantly enhance the efficacy of cancer therapies. The ability of ECCT to induce changes in the expression of inflammatory markers, such as CCL2 and IL18, suggests that this therapy not only targets cancer cells directly but also leverage the immune system to mount a more effective anti-tumour response [8]. Research on ECCT has gained momentum in recent years, particularly due to its potential as a complementary treatment option in oncology. Early studies demonstrated that exposure to low-frequency electric fields could inhibit the growth of various cancer cell lines, including breast cancer, lung cancer, and oral squamous cell carcinoma. For instance, a study found that applying an electric field at 100 kHz effectively reduced the viability of MCF-7 breast cancer cells in vitro, showcasing the therapy’s promise as a non-invasive alternative [9]. In vivo experiments using animal models have also yielded encouraging results. One notable study involving DMBA-induced breast cancer in mice revealed that ECCT significantly reduced tumour size compared to control groups. Researchers observed not only a decrease in tumours mass but also a corresponding increase in apoptotic cells, indicating that the therapy effectively triggered cell death mechanisms [10]. Moreover, investigations into the molecular underpinnings of ECCT have revealed its potential to modulate specific signalling pathways associated with cancer progression. Previous findings suggested that ECCT could upregulate tumour suppressor genes and downregulate oncogenes, contributing to its anti-proliferative effects. The ability of ECCT to affect cytokine expression further highlights its dual action: directly targeting tumour cells while also modifying the inflammatory landscape of the tumour microenvironment [11]. Overall, the growing body of evidence supporting ECCT’s efficacy in cancer treatment underscores its potential to serve as a valuable tool in the oncologist’s arsenal. As research continues, understanding the precise mechanisms through which ECCT operates will be crucial in optimizing its application and integrating it into standard cancer care practices [12].Mechanism of ECCT
In the investigation of Noncontact Electro Capacitive Cancer Therapy (ECCT), researchers employed a well-established animal model using Sprague Dawley rats. This model is widely recognized for its relevance in studying breast cancer due to its similar physiological characteristics to humans. In this study, the rats were induced with tumours using a chemical carcinogen, 7,12-dimethylbenz[a]anthracene (DMBA), which mimics the progression of breast cancer. This approach allowed researchers to create a controlled environment to observe the effects of ECCT on tumour growth and associated inflammatory responses [13]. The experimental design involved four distinct treatment groups, each consisting of six rats. Two groups were DMBA-induced, with one receiving ECCT treatment and the other serving as a control. The remaining two groups were non-DMBA-induced rats, also divided between those receiving ECCT and those not. This comprehensive setup enabled the team to assess the effects of ECCT both on induced tumours and in a healthy context, providing valuable insights into the therapy’s effectiveness [14]. To measure tumour growth and inflammation, researchers employed several advanced techniques, including histological examination and immunohistochemistry. Tumour tissues were collected and processed for staining, which allowed for detailed visualization of cellular structures and the presence of specific proteins [14]. Immunohistochemistry, in particular, was instrumental in assessing the expression levels of markers associated with cell proliferation and apoptosis, such as PCNA (proliferating cell nuclear antigen) and Caspase3. The study’s findings revealed a significant impact of ECCT on tumour growth in the treated rats. Although the overall size of the tumours did not decrease dramatically, notable differences in tumour texture and cellular composition were observed. The tumours in the ECCT-treated group exhibited signs of necrosis and a softer consistency, suggesting altered growth dynamics compared to the control group. This change implies that while the treatment may not directly reduce tumour size, it effectively influences the tumour environment, potentially inhibiting further growth [15]. In terms of protein expression, ECCT treatment led to decreased levels of PCNA and ErbB2, both of which are associated with tumour proliferation. Conversely, markers of apoptosis, such as Caspase3, were significantly increased in the treated tumours. Additionally, the presence of CD68, a marker for macrophages, was elevated, indicating enhanced immune activity within the tumour microenvironment. These changes highlight ECCT’s dual role in targeting cancer cells directly while also promoting an immune response [16]. Gene expression analysis further underscored the therapy’s effectiveness, showing significant differences in the expression of inflammatory markers CCL2 and IL18 between treated and untreated tumours. In the ECCT-treated group, the expression of both CCL2 and IL18 was downregulated, suggesting a reduction in the inflammatory signalling that often supports tumour growth. These findings provide compelling evidence that ECCT not only inhibits tumour cell proliferation but also modifies the inflammatory landscape of breast cancer, paving the way for potential new strategies in cancer therapy [17]. The results of research on ECCT provide compelling evidence for the efficacy of Noncontact Electro Capacitive Cancer Therapy (ECCT) in influencing tumour dynamics in breast cancer models. The observed reduction in tumour proliferation, indicated by decreased levels of PCNA and ErbB2, alongside increased apoptosis marked by elevated Caspase3, underscores the potential of ECCT to shift the balance between cell growth and cell death. This shift is critical in cancer treatment, as a primary goal of therapy is to halt the proliferation of malignant cells while promoting their elimination [18]. The findings suggest that ECCT can disrupt the signalling pathways that drive tumour growth, potentially leading to better patient outcomes. One of the significant insights from thee research is the role of inflammatory markers, particularly CCL2 and IL18, in the tumour microenvironment. Both of these cytokines have been implicated in cancer progression. CCL2, also known as monocyte chemoattractant protein-1, is a potent attractant for monocytes and macrophages. It plays a vital role in the recruitment of immune cells to the tumour site, where they can either promote tumour growth through inflammation or contribute to tumour suppression. Elevated levels of CCL2 have been associated with increased metastasis and poor prognosis in breast cancer patients. The downregulation of CCL2 in tumours treated with ECCT indicates that the therapy may help mitigate the inflammatory environment that supports tumour progression [18]. Similarly, IL18 has been shown to have a dual role in cancer, acting as both a promoter of immune responses and a facilitator of tumour growth. High levels of IL18 can enhance cancer cell proliferation and invasiveness, making it a key player in metastasis. The observed decrease in IL18 expression following ECCT treatment suggests that this therapy could effectively reduce the inflammatory signals that encourage tumour advancement, providing another layer of therapeutic benefit [19]. The implications of these findings for future cancer therapies are substantial. The ability of ECCT to induce apoptosis and alter the expression of key inflammatory markers like CCL2 and IL18 positions it as a promising adjunct therapy in oncology [19]. As cancer treatment continues to evolve, incorporating therapies that not only target the tumour but also modulate the immune response and inflammatory environment may lead to more effective treatment regimens. Moreover, the non-invasive nature of ECCT makes it particularly attractive for clinical application, as it may reduce the side effects associated with traditional therapies. Future research could focus on integrating ECCT with existing treatment modalities, such as chemotherapy or immunotherapy, to enhance overall effectiveness. By understanding the precise mechanisms through which ECCT operates, researchers can optimize its application, potentially leading to improved outcomes for patients facing the challenges of cancer [20].Conclusion
This blog underscores the significant potential of Noncontact Electro Capacitive Cancer Therapy (ECCT) as a novel approach to cancer treatment, particularly in the context of breast cancer. By demonstrating that ECCT can effectively reduce tumour proliferation and enhance apoptosis through the modulation of inflammatory markers such as CCL2 and IL18, the research provides compelling evidence for its role in altering the tumour microenvironment. These findings suggest that ECCT not only targets cancer cells directly but also influence the surrounding immune landscape, offering a multifaceted strategy to combat malignancies. The implications of this blog extend to clinical settings, where ECCT presents a promising adjunct therapy. Its non-invasive nature could lead to fewer side effects compared to traditional treatments, making it an appealing option for patients who may be seeking alternatives or supplementary therapies to standard care. Integrating ECCT into existing treatment protocols could enhance overall treatment efficacy, particularly in cases where inflammatory pathways contribute to tumor growth and metastasis. However, to fully realize the potential of ECCT in clinical practice, further research and clinical trials are essential. Future studies should aim to validate these findings in larger, diverse populations and explore the long-term effects of ECCT on patient outcomes. Additionally, investigating the mechanisms by which ECCT modulates immune responses will provide deeper insights into its therapeutic effects. In conclusion, this research opens new avenues for cancer therapy, highlighting the need for continued exploration of innovative treatment strategies like ECCT. As we advance our understanding of its mechanisms and efficacy, we can move closer to integrating this promising therapy into routine cancer care, ultimately improving the lives of patients facing the challenges of cancer.References
- Alamsyah F, Ajrina IN, Dewi FNA, et al.: Antiproliferative Effect of Electric Fields on Breast Tumor Cells In Vitro and In Vivo. Indones J Cancer Chemoprevent. 2015;6(3):7 10.14499/indonesianjcanchemoprev6iss3pp71-77
- Mujib SA, Alamsyah F, Taruno WP: Cell Death and Induced p53 Expression in Oral Cancer, HeLa, and Bone Marrow Mesenchyme Cells under the Exposure to Noncontact Electric Fields. Integr Med Int.2017;4(3–4):161–70. 10.1159/000485186
- Kirson ED, Gurvich Z, Schneiderman R, et al.: Disruption of cancer cell replication by alternating electric fields. Cancer Res.2004;64(9):3288–95. 10.1158/0008-5472.CAN-04-0083
- Kirson ED, Dbalý V, Tovarys F, et al.: Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A.2007;104(24):10152–7. 10.1073/pnas.0702916104
- Giladi M, Schneiderman RS, Voloshin T, et al.: Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells. Sci Rep.2015;5:18046. 10.1038/srep18046
- Timaner M, Beyar-Katz O, Shaked Y: Analysis of the Stromal Cellular Components of the Solid Tumor Microenvironment Using Flow Cytometry. Curr Protoc Cell Biol.2016;70:19.8.1–19.18.12. 10.1002/0471143030.cb1918s70
- Kitamura T, Qian BZ, Soong D, et al.: CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med.2015;212(7):1043–59. 10.1084/jem.20141836
- Kobori T, Hamasaki S, Kitaura A, et al.: Interleukin-18 Amplifies Macrophage Polarization and Morphological Alteration, Leading to Excessive Angiogenesis. Front Immunol.2018;9:334. 10.3389/fimmu.2018.00334
- Hoare JI, Rajnicek AM, McCaig CD, et al.: Electric fields are novel determinants of human macrophage functions. J Leukoc Biol.2016;99(6):1141–51. 10.1189/jlb.3A0815-390R
- Park JI, Song KH, Jung SY, et al.: Tumor-Treating Fields Induce RAW264.7 Macrophage Activation Via NK-κB/MAPK Signaling Pathways. Technol Cancer Res Treat.2019;18:1533033819868225. 10.1177/1533033819868225
- Yang Y, Cheon S, Jung MK, et al.: Interleukin-18 enhances breast cancer cell migration via down-regulation of claudin-12 and induction of the p38 MAPK pathway. Biochem Biophys Res Commun.2015;459(3):379–86. 10.1016/j.bbrc.2015.02.108
- Menegatti S, Bianchi E, Rogge L: Anti-TNF Therapy in Spondyloarthritis and Related Diseases, Impact on the Immune System and Prediction of Treatment Responses. Front Immunol.2019;10:382. 10.3389/fimmu.2019.00382
- Yu PF, Huang Y, Han YY, et al.: TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2 +neutrophils. Oncogene. 2017;36(4):482–490. 10.1038/onc.2016.217
- Zhang H, Liu K, Xue Z, et al.: High-voltage pulsed electric field plus photodynamic therapy kills breast cancer cells by triggering apoptosis. Am J Transl Res.2018;10(2):334–351.
- Li K, Wei L, Huang Y, et al.: Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int J Oncol.2016;48(6):2479–87. 10.3892/ijo.2016.3483
- Putra A, Ridwan FB, Putridewi AI, et al.: The role of tnf-α induced mscs on suppressive inflammation by increasing tgf-β and il-10. Open Access Maced J Med Sci.2018;6(10):1779–1783. 10.3889/oamjms.2018.404
- Hao NB, Lu MH, Fan YH, et al.: Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol.2012;2012:948098. 10.1155/2012/948098
- Langowski JL, Kastelein RA, Oft M: Swords into plowshares: IL-23 repurposes tumor immune surveillance. Trends Immunol.2007;28(5):207–12. 10.1016/j.it.2007.03.006
- Lee C, Jeong H, Bae Y, et al.: Targeting of M2-like tumor-associated macrophages with a melittin-based pro-apoptotic peptide. J Immunother Cancer.2019;7(1):1–14. 10.1186/s40425-019-0610-4
- Fasoulakis Z, Kolios G, Papamanolis V, et al.: Interleukins Associated with Breast Cancer. Cureus.2018;10(11):e3549. 10.7759/cureus.3549