Introduction
Breast cancer is one of the most prevalent cancers globally, affecting millions of women and representing a leading cause of cancer-related mortality. In 2020 alone, approximately 2.3 million women were diagnosed with breast cancer, making it the most common cancer worldwide. The disease is particularly concerning due to its complex nature and the myriad factors contributing to its progression, including genetics, lifestyle, and environmental influences. Early detection and advancements in treatment have improved survival rates, yet breast cancer remains a significant health issue, particularly as some patients experience recurrence or metastasis [1]. Despite progress in treatment strategies, existing modalities face numerous challenges. Surgical interventions, such as lumpectomy and mastectomy, while effective in removing tumors, can lead to significant physical and psychological impacts on patients. Systemic therapies, including chemotherapy and targeted therapies, are often accompanied by severe side effects, such as nausea, fatigue, and immunosuppression, which can severely diminish a patient’s quality of life [2]. Furthermore, issues like multi-drug resistance can arise, rendering standard treatments ineffective for some patients. These challenges highlight the urgent need for novel therapeutic approaches that can complement or improve upon current treatment modalities [3]. In response to these needs, researchers are exploring innovative therapies that harness the body’s immune response to combat cancer. One such promising approach is Electro-Capacitive Cancer Therapy (ECCT). This therapy utilizes low-intensity, non-contact electric fields to target cancer cells without directly affecting surrounding healthy tissues. By exposing tumors to carefully controlled electric fields, ECCT aims to disrupt cancer cell proliferation and activate immune responses [4]. Initial studies have shown that ECCT not only inhibits tumor growth but also enhances the activity of cytotoxic T cells, which play a crucial role in the immune system’s ability to eliminate cancerous cells. This novel approach holds the potential to improve outcomes for breast cancer patients while minimizing the adverse effects associated with conventional therapies [5]. As we delve deeper into the research on ECCT, we will explore its mechanisms of action, the results of recent studies, and its implications for the future of cancer treatment. In order to understand the importance of the CD4/CD8 ratio and T cell activation. CD4+ T cells, known as helper T cells, play a vital role in orchestrating the immune response, activating other immune cells, including CD8+ T cells, which are cytotoxic and directly target and kill cancer cells [6]. The ratio of CD4 to CD8 T cells can provide valuable insights into the immune status of an individual, with a higher ratio often indicating a robust immune response. Conversely, a decreased CD4/CD8 ratio can suggest immune suppression, which is commonly seen in cancer patients. Therefore, understanding these dynamics is essential for assessing the effectiveness of ECCT and its impact on the immune landscape in the tumor microenvironment [7]. The experimental designs in the past research on ECCT in breast cancer involved dividing the rats into four groups: a control group that did not receive induction or therapy, a placebo group receiving sham therapy, a group induced with tumors but not treated, and a group that received ECCT. Each group was monitored for tumor growth over a specified period, and this design enabled researchers to draw meaningful comparisons between the effects of ECCT and traditional treatment methods [8]. To measure tumor growth and immune responses, several advanced techniques were employed. Histological examination allowed for the assessment of tumor morphology and cellular changes, while immunohistochemistry was used to identify specific protein expressions related to cell proliferation and apoptosis, such as PCNA (proliferating cell nuclear antigen), ErbB2, and Caspase3 [9]. Additionally, the distributions of immune cells, including CD4+ and CD8+ T cells, were analyzed to gauge the immune response within the tumor environment [10]. The findings from the available research highlighted the significant impact of ECCT on tumor growth rates. Rats treated with ECCT exhibited notably lower tumor growth compared to those in the untreated groups, suggesting that ECCT can effectively inhibit tumor progression. This aligns with the goal of developing non-invasive therapies that can provide similar or enhanced outcomes compared to traditional cancer treatments [11]. In terms of cellular responses, changes in protein expression were observed. A decrease in PCNA and ErbB2 levels in tumors treated with ECCT indicated reduced proliferation and possibly a shift toward apoptotic pathways, as evidenced by increased Caspase3 expression. This highlights ECCT’s potential to not only slow tumor growth but also to induce cancer cell death [12]. Furthermore, observations of T cell distributions revealed an interesting dynamic in the immune response. The treated group showed an increased presence of CD8+ T cells, which are crucial for direct tumor cell elimination [13]. Conversely, the CD4/CD8 ratio was found to be lower in the ECCT-treated group compared to untreated counterparts, suggesting a shift in immune activity that may favor more aggressive targeting of the tumor [14]. These findings underscore the importance of integrating immune response assessments into cancer therapy research, particularly for innovative approaches like ECCT. As the scientific community continues to explore the intricacies of cancer treatment, understanding the relationship between therapies, immune activation, and tumor dynamics will be crucial in developing more effective strategies for managing breast cancer and other malignancies [15]. The results from multiple studies reveal significant insights into the effects of Electro-Capacitive Cancer Therapy (ECCT) on mammary tumors and the underlying immune mechanisms. The observed reduction in tumor proliferation, coupled with an increase in apoptosis, highlights the potential of ECCT as an effective therapeutic strategy against breast cancer [16].Interpretation of Reduced Tumor Proliferation and Increased Apoptosis
The decrease in tumor proliferation, as indicated by lower levels of PCNA and ErbB2, suggests that ECCT effectively disrupts the mechanisms driving cancer cell growth. This is particularly noteworthy because ErbB2 is often overexpressed in aggressive breast cancers and is associated with poor prognosis. By targeting the proliferation pathways, ECCT may help slow down tumor progression and potentially prevent metastasis [17]. Increased apoptosis, marked by elevated Caspase3 levels, further reinforces the therapeutic potential of ECCT. Apoptosis is a critical process in eliminating dysfunctional cells, and its induction in tumor cells signifies a positive treatment outcome. The presence of apoptotic bodies and the mechanisms leading to programmed cell death could be pivotal in not only shrinking the tumor size but also preventing the recurrence of cancerous growth. This dual effect—slowing proliferation while promoting cell death—positions ECCT as a compelling option in the landscape of cancer therapies [18].Role of CD8+ T Cells and the Significance of Decreased CD4/CD8 Ratio in Tumor Inhibition
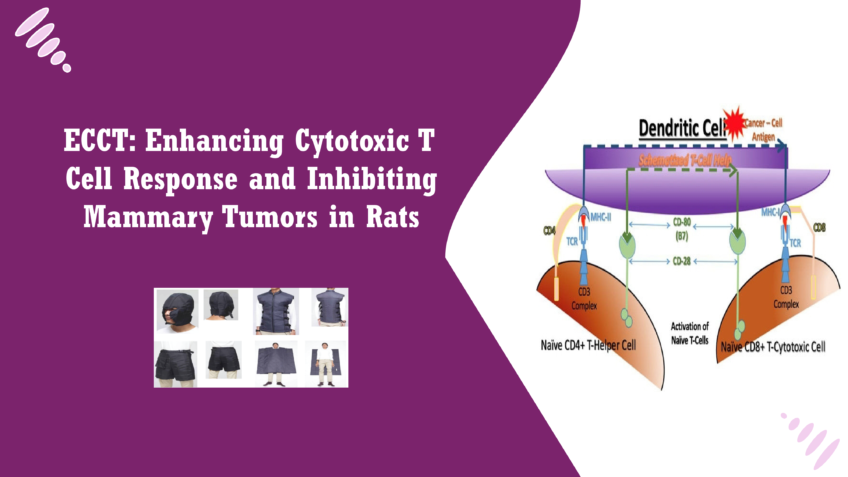
The increased presence of CD8+ T cells in the tumors of rats treated with ECCT suggests that this therapy enhances cytotoxic immune responses. CD8+ T cells play a crucial role in identifying and destroying tumor cells, and their activation is essential for an effective anti-tumor response. The ability of ECCT to recruit and activate these cells indicates that it not only acts directly on the tumor but also bolsters the immune system’s capacity to combat cancer. Conversely, the observed decrease in the CD4/CD8 ratio in the ECCT-treated group is noteworthy. A lower CD4/CD8 ratio can indicate a shift in immune dynamics, suggesting that the immune response is becoming more cytotoxic in nature. While CD4+ T cells are important for orchestrating the immune response, a predominance of CD8+ T cells can be indicative of a more aggressive anti-tumor immunity. This shift is significant, as it may imply that ECCT is not just enhancing immune surveillance but also fine-tuning the immune response to favor direct tumor cell elimination [19].Potential Mechanisms by Which ECCT Modulates Immune Responses
ECCT’s ability to modulate immune responses may stem from several interrelated mechanisms. One hypothesis is that the electric fields alter the tumor microenvironment, making it more conducive to immune cell infiltration. Electric fields can influence cellular signaling pathways, potentially enhancing the migration of immune cells such as T cells and macrophages to the tumor site. This enhanced infiltration can facilitate a stronger immune response against the tumor. Additionally, the exposure to ECCT may induce changes in the expression of various cytokines and chemokines, further promoting the recruitment and activation of immune cells. For example, studies suggest that electric fields can enhance the production of pro-inflammatory cytokines, which play a crucial role in activating immune responses. The presence of these signaling molecules could improve the communication between immune cells, fostering a more coordinated attack on tumor cells [20]. Moreover, the potential for ECCT to induce immunogenic cell death could play a role in shaping the immune landscape. When tumor cells die in an immunogenic manner, they can release antigens that enhance the activation of T cells. This process could explain the observed increase in CD8+ T cell activity and the associated tumor inhibition. This highlights the multifaceted nature of ECCT’s effects on tumor growth and immune modulation. By understanding these mechanisms, researchers can better appreciate the potential of ECCT as a complementary therapy in breast cancer treatment, paving the way for future studies that may lead to innovative clinical applications.Conclusion
In summary, this blog highlights the significant promise of Electro-Capacitive Cancer Therapy (ECCT) as an innovative approach to managing breast cancer. By demonstrating its ability to reduce tumor proliferation and increase apoptosis, along with enhancing the activation of CD8+ T cells, ECCT showcases its potential not only as a tumor-inhibiting therapy but also as a strategy to modulate the immune response in favor of combating cancer. The future potential for ECCT in clinical settings is considerable. Given its non-invasive nature and the lack of severe side effects associated with its application, ECCT could serve as a valuable adjunct to existing cancer therapies. Its ability to target tumors while simultaneously activating the immune system positions it as a promising candidate for inclusion in multidisciplinary treatment plans, potentially improving patient outcomes and quality of life. However, to fully realize the benefits of ECCT, further research and clinical trials are essential. Continued exploration will help establish optimal treatment protocols, identify patient populations that may benefit most, and elucidate the mechanisms underlying its effectiveness. By advancing our understanding of ECCT, we can pave the way for its integration into standard cancer care, offering hope to those affected by breast cancer and potentially transforming the landscape of oncology.References:
- Siegel R, Naishadham D, Jemal A: Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. 10.3322/caac.20138
- Bray F, Ferlay J, Soerjomataram I, et al.: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492
- Moo TA, Sanford R, Dang C, et al.: Overview of breast cancer therapy. PET Clin. 2018;13(3):339–354. 10.1016/j.cpet.2018.02.006
- Andersen K, Kehlet H: Persistent pain after breast cancer treatment: a critical review of risk factor and strategies for prevention. J Pain. 2011;12(7):725–746. 10.1016/j.jpain.2010.12.005
- Bukowski K, Kciuk M, Kontek R: Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21(9):3233. 10.3390/ijms21093233
- Conze D, Weiss L, Regen P, et al.: Autocrine production of interleukin 6 causes multidrug resistance in breast cancer cells. Cancer Res. 2001;61(24):8851–8858.
- Kumar M, Nagpal R, Hemalatha R, et al.: Targeted cancer therapies: the future of cancer treatment. Acta Biomed. 2012;83(3):220–233.
- Bokstein F, Blumenthal D, Limon D, et al.: Concurrent tumor treating Fields (TTFields) and radiation therapy for newly diagnosed glioblastoma: a prospective safety and feasibility study. Front Oncol. 2020;10:411. 10.3389/fonc.2020.00411
- Kirson ED, Schneiderman RS, Dbalý V, et al.: Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med Phys. 2009;9:1. 10.1186/1756-6649-9-1
- Jo Y, Hwang SG, Jin YB, et al.: Selective toxicity of tumor treating fields to melanoma: an in vitro and in vivo study. Cell Death Discov. 2018;4:46. 10.1038/s41420-018-0106-x
- Alamsyah F, Ajrina IN, Dewi FN, et al.: Antiproliferative effect of electric fields on breast tumor cells in vitro and in vivo. Indones J Cancer Chemoprev. 2015;6(3):71–77. 10.14499/indonesianjcanchemoprev6iss3pp71-77
- Gonzalez H, Hagerling C, Werb Z: Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–1284. 10.1101/gad.314617.118
- Maimela NR, Liu S, Zhang Y: Fates of CD8+ T cells in tumor microenvironment. Comput Struct Biotechnol J. 2019;17:1–13. 10.1016/j.csbj.2018.11.004
- Yang M, Li Z, Ren M, et al.: Stromal infiltration of tumor-associated macrophages conferring poor prognosis of patients with basal-like breast carcinoma. J Cancer. 2018;9(13):2308–2316. 10.7150/jca.25155
- Medrek C, Ponten F, Jirstrom K, et al.: The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. 10.1186/1471-2407-12-306
- Donzelli S, Milano E, Pruszko M, et al.: Expression of ID4 protein in breast cancer cells induces reprogramming of tumour associated macrophages. Breast Cancer Res. 2018;20(1):59. 10.1186/s13058-018-0990-2
- Dimberu PM, Leonhardt RM: Cancer immunotherapy takes a multi-faceted approach to kick the immune system into gear. Yale J Biol Med. 2011;84(4):371–380.
- Petri AK, Schmiedchen K, Stunder D, et al.: Biological Effects of Exposure to Static Electric Fields in Humans and Vertebrates: A Systematic Review. Environ Health. 2017;16(1):41. 10.1186/s12940-017-0248-y
- Arnold CE, Rajnicek AM, Hoare JI, et al.: Physiological strength electric fields modulate human T cell activation and polarisation. Sci Rep. 2019;9(1):17604. 10.1038/s41598-019-53898-5
- Hoare JI, Rajnicek AM, McCaig CD, et al.: Electric fields are novel determinants of human macrophage functions. J Leukoc Biol. 2016;99(6):1141–51. 10.1189/jlb.3A0815-390R