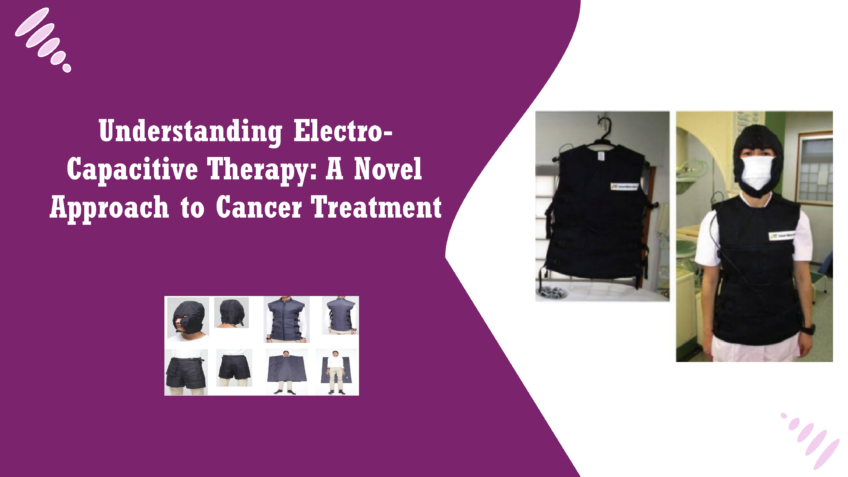
What is Electro-Capacitive Therapy?
Electro-Capacitive Therapy (ECT) is a non-invasive therapeutic technique that leverages electrical fields to treat various diseases, with a growing focus on its application in cancer treatment. It involves the use of capacitive electrical fields to disrupt cellular activity, particularly targeting cancer cells while sparing healthy tissues. ECT works by placing electrodes on the body, which emit low-level electrical pulses designed to interact with cells at the molecular level. This interaction has shown the potential to inhibit cancer cell growth and promote their destruction [1].How ECT is emerging as a novel cancer treatment
In the realm of oncology, Electro-Capacitive Therapy is gaining attention as an innovative approach to tackling cancer. Traditional cancer treatments like chemotherapy, radiation, and surgery can have significant side effects, often harming healthy cells in the process. ECT offers a potentially safer alternative by selectively disrupting cancer cell function. Early studies and clinical trials suggest that ECT may enhance the precision of cancer treatment, reduce damage to healthy tissue, and lessen the side effects associated with traditional therapies. Because of this, ECT is increasingly being explored as part of a multimodal treatment strategy, potentially used in conjunction with existing therapies [2].The science behind using electric fields in medical therapies
The use of electric fields in medical treatments is not a new concept. For decades, electric fields have been used to promote tissue regeneration, wound healing, and pain management. In the context of cancer, electric fields exert their effects by influencing the electrical properties of cells. Cancer cells, due to their high rate of division, are particularly sensitive to external electric fields. ECT harnesses this sensitivity by applying low-intensity, alternating electric fields that interfere with key cellular processes such as mitosis (cell division). This disruption not only prevents the proliferation of cancer cells but may also lead to their destruction, through mechanisms like apoptosis (programmed cell death), making ECT a promising addition to the arsenal of cancer therapies [3]. This novel approach stems from a deeper understanding of bioelectricity—how cells communicate and function using electrical signals. As research continues to uncover the nuances of ECT’s mechanisms, its role in the future of cancer therapy looks increasingly promising.How Does Electro-Capacitive Therapy Work?
Electro-Capacitive Therapy (ECT) operates on the principle of capacitive electrical fields, which are formed between two conductive objects (electrodes) that create a non-contact interaction with biological tissues. When an alternating electric current is applied between these electrodes, it generates an oscillating electric field that interacts with the cells in the tissue, without penetrating the skin directly. This is a key aspect of capacitive coupling—where energy is transferred without the need for direct electrical conduction. In a biological context, capacitive fields influence cells by altering the distribution of electrical charges on their membranes. Cells, especially rapidly dividing ones like cancer cells, are highly sensitive to these external electrical environments. The oscillating electric fields in ECT can modify cellular functions by disrupting the normal electrochemical gradients across cell membranes, affecting the stability and functionality of cancer cells [4]. Cancer cells differ from normal cells in that they proliferate uncontrollably and have a unique cellular environment. One of the critical stages of cancer progression is mitosis, where cancer cells divide and multiply at an accelerated rate. ECT leverages the sensitivity of these dividing cells to electric fields. When cancer cells attempt to undergo mitosis, the applied capacitive electrical fields interfere with their ability to align and segregate chromosomes properly. This disruption can cause significant structural stress within the cancer cells, leading to multiple outcomes: the cell cycle may be halted (inhibiting further cell division), or the cells may undergo apoptosis (programmed cell death) because of the inability to maintain normal cellular processes. Essentially, the capacitive fields “confuse” the cancer cells’ internal signaling and regulatory mechanisms, leading to their destruction or preventing their growth.How ECT targets cancer cells without harming healthy cells
One of the remarkable features of Electro-Capacitive Therapy is its ability to target cancer cells specifically, while minimizing damage to healthy tissue. This selectivity arises from two main factors: the nature of cancer cells and the frequency of the applied electric fields. Cancer cells are particularly vulnerable to ECT because they exhibit a high rate of division and have abnormal membrane properties. Their rapid mitotic activity makes them more responsive to the effects of external electric fields, which disrupt the mitosis process. In contrast, most healthy cells divide at a much slower rate, and their normal membrane potential allows them to be less affected by these external fields. Moreover, the parameters of ECT—such as the frequency and intensity of the electric fields—are optimized to specifically disrupt the behavior of cancer cells. Healthy cells, which are in a more stable, non-dividing state, do not experience the same level of stress from the electric fields, allowing them to continue functioning normally. This selectivity is a key advantage of ECT, as it minimizes the collateral damage typically associated with traditional cancer treatments like chemotherapy and radiation, which can harm both healthy and cancerous cells. In summary, ECT works by creating electrical fields that selectively interfere with the abnormal processes in cancer cells while sparing healthy cells, making it a highly targeted and potentially safer cancer treatment option [5].Mechanisms of Action in Cancer Treatment
Mitosis, the process of cell division, is a key target in Electro-Capacitive Therapy (ECT). Cancer cells are characterized by their rapid and uncontrolled division, making them particularly susceptible to interventions that disrupt mitosis. During mitosis, cells go through a series of well-coordinated phases that involve DNA replication, chromosome alignment, and eventual division into two daughter cells. For this process to occur smoothly, cancer cells must maintain proper electrical and structural integrity. ECT interferes with mitosis by applying alternating electrical fields that disrupt the proper alignment and separation of chromosomes. Specifically, ECT affects the mitotic spindle—a structure essential for guiding chromosomes during cell division. The capacitive electrical fields generated in ECT cause physical stress on the spindle’s microtubules, preventing chromosomes from aligning correctly at the cell’s equatorial plate. This misalignment forces the cell to either delay mitosis or undergo abnormal division, both of which can lead to cell cycle arrest (where the cell cannot continue dividing) or eventual cell death. The inability to complete mitosis properly is devastating for cancer cells, as they depend on rapid division to fuel tumor growth [6].The impact on cancer cell growth and metastasis
By disrupting mitosis, ECT directly impacts cancer cell growth. If cancer cells cannot divide successfully, they are unable to proliferate, which halts tumor expansion. Additionally, the stress placed on dividing cancer cells by ECT induces abnormal division, leading to the generation of dysfunctional daughter cells. These damaged cells may either be incapable of further division or may undergo apoptosis (programmed cell death). ECT also has potential implications for metastasis, the process by which cancer spreads from one part of the body to another. Metastasis depends on the ability of cancer cells to proliferate, invade surrounding tissues, and migrate to distant organs. By interfering with the cell division process, ECT limits the pool of cancer cells capable of contributing to metastatic spread. Furthermore, the destruction of cells through apoptosis or impaired division reduces the likelihood of aggressive cancer cells breaking away from the primary tumor and forming secondary tumors in other parts of the body. Though research on ECT’s specific effects on metastasis is still ongoing, the disruption of mitosis offers a promising mechanism for reducing both local tumor growth and distant metastasis. Apoptosis, or programmed cell death, is a controlled process by which cells self-destruct when they are damaged or no longer needed. ECT can trigger apoptosis in cancer cells through several mechanisms. First, the disruption of mitosis caused by ECT creates significant internal stress in the cell. When cancer cells fail to divide properly or sustain damage during attempted division, they may activate apoptotic pathways as a safeguard against the propagation of defective cells [7]. The electrical fields used in ECT alter the cell’s internal environment, including the electrical potential of the cell membrane, which can trigger signaling pathways that lead to apoptosis. Mitochondria, the energy-producing organelles within cells, are also affected by these electrical disturbances. Mitochondrial damage can release pro-apoptotic factors like cytochrome c into the cytoplasm, further initiating the cascade of events that result in cell death. Apoptosis ensures that damaged or dysfunctional cancer cells are eliminated in a controlled manner, without triggering the inflammatory response that is associated with necrosis (uncontrolled cell death). The induction of apoptosis through ECT provides a targeted means of killing cancer cells while sparing healthy cells, which typically do not experience the same level of mitotic stress or membrane destabilization. In summary, ECT’s mechanisms of action in cancer treatment include the disruption of mitosis, inhibition of cancer cell proliferation, reduction of metastatic potential, and the induction of apoptosis. By leveraging these mechanisms, ECT offers a novel and targeted approach to suppressing tumor growth and improving cancer outcomes [8].Comparing Electro-Capacitive Therapy to Traditional Treatments
How ECT differs from chemotherapy, radiation, and surgery
Electro-Capacitive Therapy (ECT) stands apart from traditional cancer treatments like chemotherapy, radiation, and surgery due to its non-invasive, targeted approach. Chemotherapy works by administering drugs that kill rapidly dividing cells, affecting both cancerous and healthy cells, leading to widespread systemic effects such as hair loss, immune suppression, and gastrointestinal issues. Radiation therapy uses high-energy beams to destroy cancer cells but can also damage surrounding healthy tissues. Surgery, while effective in removing localized tumors, is invasive and can lead to complications such as infections, pain, and recovery downtime. ECT, on the other hand, utilizes low-intensity alternating electric fields applied externally through electrodes, targeting cancer cells selectively without directly damaging healthy tissues. Unlike chemotherapy or radiation, ECT doesn’t introduce harmful chemicals or ionizing radiation into the body. Instead, it relies on the unique electrical properties of cancer cells, which are more susceptible to the disruption of their division process due to their high mitotic activity. The non-invasive nature of ECT makes it a potentially safer option for patients, with fewer immediate risks compared to surgery [9].Potential advantages such as reduced side effects
One of the most significant advantages of Electro-Capacitive Therapy is its potential for reduced side effects. Traditional treatments often come with a host of adverse reactions due to their impact on healthy cells. Chemotherapy, for example, affects rapidly dividing cells throughout the body, leading to toxicity in the bone marrow, digestive system, and hair follicles. Radiation can cause skin burns, fatigue, and damage to nearby organs. Surgery, while effective in removing tumors, can result in physical trauma and extended recovery times. In contrast, ECT’s selective targeting of cancer cells results in fewer side effects. The electrical fields used in ECT are tuned to disrupt cancer cell division while leaving healthy, non-dividing cells relatively unharmed. This selectivity means that ECT can potentially be applied over longer periods with minimal discomfort to the patient, and without the harsh systemic effects seen with chemotherapy and radiation. This also makes ECT an attractive option for patients who may not tolerate conventional therapies well, such as the elderly or those with pre-existing conditions.Addressing the combination of ECT with other treatments
ECT is not intended to replace traditional treatments entirely but can be used in combination with them to enhance overall therapeutic efficacy. Combining ECT with chemotherapy or radiation has the potential to increase the effectiveness of these treatments while reducing their required doses. This strategy could allow for a more tailored approach to cancer care, where patients receive lower doses of chemotherapy or radiation, thereby minimizing side effects while maintaining therapeutic benefit. Additionally, ECT may serve as an adjunct to surgery. For instance, ECT could be used post-operatively to target any remaining cancer cells that surgery may have missed, reducing the risk of recurrence. This multimodal approach is an area of growing interest in oncology, as combining treatments that work through different mechanisms—such as disrupting mitosis (ECT), damaging DNA (chemotherapy/radiation), or physically removing tumors (surgery)—can provide a more comprehensive attack on cancer.Clinical Implications and Benefits of Electro-Capacitive Therapy
Preliminary clinical studies and trials investigating ECT’s use in cancer treatment have shown promising results. Early trials have focused on the safety and efficacy of ECT in various cancer types, including glioblastoma, pancreatic cancer, and lung cancer. These studies reveal that ECT can slow tumor progression and, in some cases, reduce tumor size without causing significant harm to healthy tissues. Patients undergoing ECT have reported fewer side effects compared to traditional therapies, supporting the notion that ECT is a gentler yet effective cancer treatment. Furthermore, animal studies and in vitro experiments have demonstrated ECT’s capacity to interfere with cancer cell growth and induce apoptosis in a wide range of tumor types. These studies provide a strong foundation for more extensive clinical trials to determine the optimal application of ECT in humans, as well as its long-term effectiveness in preventing cancer recurrence. ECT holds considerable potential for improving patient outcomes, particularly in terms of quality of life. By minimizing the collateral damage to healthy tissues, ECT allows patients to undergo treatment without experiencing the debilitating side effects commonly associated with chemotherapy and radiation. This improvement in tolerability means that patients may remain more active and retain a better overall quality of life during treatment. In terms of reducing cancer recurrence, ECT’s ability to target residual cancer cells left behind after surgery or other treatments could play a significant role. Residual microscopic cancer cells are often responsible for cancer recurrence, and ECT’s selective targeting of rapidly dividing cells makes it a promising tool for eradicating these lingering threats. By reducing the risk of recurrence, ECT could improve long-term patient survival rates and provide a durable cancer treatment option [10]. As research into ECT continues, there is increasing interest in its application across a wide range of cancer types. Initial studies have focused primarily on cancers of the brain, such as glioblastoma, due to the challenges these cancers pose for conventional treatments. However, ECT’s versatility has sparked interest in its use for other difficult-to-treat cancers, including pancreatic cancer, liver cancer, and metastatic cancers. The non-invasive nature of ECT, combined with its low risk of side effects, makes it an attractive option for cancers that are either inoperable or located in sensitive areas where surgery and radiation could cause significant harm. As clinical trials expand and more data is collected, ECT could become a widely accepted treatment modality, particularly for cancers with limited therapeutic options or for patients seeking alternatives to traditional treatments.Challenges and Future Directions in Cancer Treatment
While Electro-Capacitive Therapy (ECT) shows great promise, several challenges must be addressed before it can become a mainstream cancer treatment. One of the main obstacles is accessibility. ECT technology is still in its early stages of adoption, and not all medical facilities have the necessary equipment or trained personnel to administer the therapy. As a novel treatment, ECT requires specialized devices to generate and apply the electric fields, and these devices are not yet widely available in clinics and hospitals. Cost is another significant challenge. ECT’s development, testing, and approval processes are ongoing, which adds to the initial expense of implementing the therapy. The need for advanced equipment and highly trained medical staff could drive up the costs for patients and healthcare systems, especially in the early years of its adoption. To make ECT accessible to a broader patient population, efforts will be needed to streamline production, reduce equipment costs, and integrate the therapy into existing cancer treatment frameworks. Research into ECT is rapidly expanding as scientists and clinicians work to optimize the therapy’s effectiveness across different cancer types. Ongoing clinical trials are exploring its use in combination with other cancer treatments, such as chemotherapy, radiation, and immunotherapy. These studies aim to determine how best to integrate ECT into current cancer care protocols to improve outcomes and reduce side effects. Moreover, researchers are investigating the potential for ECT to be customized based on the specific characteristics of a patient’s cancer. Personalized approaches, such as tailoring the frequency and intensity of the electric fields to match the unique properties of the tumor, could maximize treatment efficacy. There is also growing interest in using ECT to treat other conditions beyond cancer, such as benign tumors or neurological disorders, given its ability to selectively target cells with abnormal activity. Looking further into the future, advancements in bioelectronic technologies could enhance ECT’s precision and effectiveness. For example, integrating ECT with real-time imaging technologies might allow physicians to monitor cancer cell response during treatment, leading to more adaptive and individualized treatment strategies. Such innovations could help expand ECT’s applications, making it a more versatile and widely used therapy.Why ECT represents a promising addition to cancer therapy:
ECT offers a unique approach to cancer treatment by targeting cancer cells with electric fields rather than relying on the chemical or radiological methods of traditional treatments. This non-invasive, selective targeting of cancer cells makes it especially promising for patients who cannot tolerate the side effects of chemotherapy or radiation or those whose tumors are inoperable. The ability of ECT to interfere with cancer cell division and trigger apoptosis (programmed cell death) without harming healthy cells sets it apart from many existing treatments. Additionally, ECT’s potential for use in combination with other therapies makes it an attractive option in the fight against cancer. By enhancing the efficacy of conventional treatments while reducing side effects, ECT represents a promising addition to the evolving landscape of cancer therapy. Its ability to selectively disrupt cancer cells’ mitotic activity could prove especially valuable in cases where traditional treatments fall short, such as in treatment-resistant cancers or recurrent tumors. Electro-Capacitive Therapy (ECT) is an emerging, non-invasive cancer treatment that uses alternating electric fields to target and disrupt cancer cell activity. Unlike traditional treatments like chemotherapy, radiation, and surgery, ECT selectively targets cancer cells without causing significant damage to healthy tissues, making it a potentially safer option with fewer side effects. Through mechanisms such as disrupting mitosis and triggering apoptosis, ECT has demonstrated promise in slowing cancer growth and reducing tumor size. Ongoing clinical trials are exploring its effectiveness across various cancer types, and researchers are investigating ways to combine ECT with other treatments to enhance overall therapeutic outcomes. Although early results are promising, continued research is essential to fully understand ECT’s long-term effectiveness and potential applications. Clinical trials will need to establish the therapy’s efficacy across a wider range of cancers, as well as its ability to improve patient survival rates and quality of life. Research into optimizing ECT protocols, developing more affordable and accessible equipment, and exploring combination therapies will be critical in making ECT a viable option for a larger population of cancer patients. Moreover, as ECT becomes more widely studied, it is vital to address challenges related to accessibility, cost, and scalability. Collaboration between researchers, healthcare providers, and manufacturers will be necessary to ensure that ECT can be made available to patients across the globe, regardless of their socioeconomic status or geographic location.How ECT could shape the future of cancer treatment:
ECT has the potential to reshape the future of cancer treatment by offering a gentler, more targeted alternative to traditional therapies. Its ability to selectively attack cancer cells while preserving healthy tissues could reduce the suffering associated with conventional treatments, improving the overall patient experience. As technology evolves and research advances, ECT could be integrated into personalized treatment plans, offering tailored therapy based on the specific characteristics of each patient’s cancer. In the coming years, ECT may become an essential part of the cancer treatment landscape, especially as healthcare systems increasingly emphasize precision medicine and minimally invasive approaches. With its ability to complement other treatments, reduce side effects, and potentially prevent cancer recurrence, Electro-Capacitive Therapy represents a significant step forward in the ongoing battle against cancer.References
- Yayasan Kanker Indonesia 2020 Hari Kanker Sedunia 2020 Retrieved from http://yayasankankerindonesia.org/article/hari-kanker-sedunia-2020-kanker-paru-diindonesia-sebabkan-lebih-dari-26-ribu-orang-meninggal.
- Yayasan Kanker Payudara Indonesia 2018 About Breast Cancer. Retrieved from https://pitapinkykpi.or.id/tentang-kanker-payudara/?lang=en
- Collett, D 2003 Modelling Survival Data in Medical Research, Second Edition. London: Chapman and Hall.
- Cox, D 1972 Regression Model and Life Table. J Roy Stat Soc B, 34, 187-202.
- SW Purnami, KD Inayati, NWW Sari, V Chosuvivatwong, H Sriplung 2016 Survival analysis of cervical cancer using stratified Cox regression AIP Conference Proceedings 1723 (1), 030018
- Treatment of Lung, Liver, Bones, and Brain Metastasized Breast Cancer using Electro-Capacitive Cancer Therapy (ECCT). Retrieved from di http://www.c-techlabs.com/treatment-of-lungliver-bones-and-brain-metastasized-breast-cancers-using-electro-capacitive-cancer-therapyecct/
- Alamsyah F, Izzatun N A, Fitriya N A D, Diah I, Silvia A P and Warsito P T 2015 Antiproliferative Effect of Electric Fields on Breast Tumor Cells in Vitro and in Vivo Indonesia Journal of Cancer Chemoprevention
- Taruno W P 2014 The 9th Proc International Congress on Medical Laser Applications German
- Imran K O, Kazim O, Meylut T 2009 Comparison Of Bayesian Survival Anaoysis and Cox Regression Analysis in Simulated and Breast Cancer Data Sets Expert Systems with Applications 36 11341-11346
- Baosheng L, Jinming Y, Mohan S, Andrew S K, Pradip P A, Zhengqian C, Rong Y, Shoufang G, Tingmang H, Yabin W, Ningsha Y, Guangde S, Liying W 2000 Comparison Of Three Treatment Options For Single Brain Metastasis From Lung Cancer Int. J. Cancer (Radiat. Oncol. Invest) 90 37–45